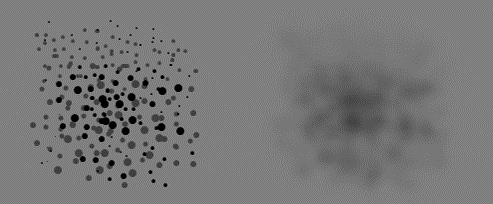
Tissue heterogeneity
As a result of the limited spatial resolution of PET scanners, the measured tissue time-activity curve (TTAC) inside a VOI (even inside single image voxel) is a mixture of the TTACs of more than one tissue component (Blomqvist et al., 1995). At its best, model parameters calculated from this kind of TTAC represents the average of true parameter values inside the VOI. Non-uniformity affects visual interpretation of diagnostic images and VOI definition (Hatt et al., 2011; Dong et al., 2015).
In PET data analysis, tissue heterogeneity is usually considered only as a nuisance, as far as it is caused by partial volume effect. But there also exists biological tissue heterogeneity. Large-scale spatial heterogeneity can even be quantified using PET, possibly providing useful data for survival analysis in oncological patients (O'Sullivan et al., 2003 and 2005; Eary et al., 2008; Asselin et al., 2012; Tixier et al., 2012; Chen et al., 2013; Hatt et al., 2013 and 2015; Willaime et al., 2013) and physiological studies of skeletal muscle (Kalliokoski et al., 2000, 2001a, 2001b, and 2003a; Heinonen et al., 2007).
Atherosclerotic vascular diseases often lead to localized tissue areas with more severe disease or local infarction.
In addition to the spatial heterogeneity observable with medical imaging, tissues are heterogeneous also on microscopic level. Animal studies with intravital multiphoton microscopy has revealed marked temporal variations at the capillary level (vasomotion), with very variable oscillation times (Aalkjær et al., 2011). Vasomotion affects the perfusion distribution in tissue, and may be associated with insulin resistance in skeletal muscle (Kusters and Barrett, 2016).
Analysis of heterogeneous data
Methods based on assumption of homogeneous tissue may lead to over- or underestimation of model parameters. Compartmental model results (even macroparameters) may be biased. Because of tissue heterogeneity, a wrong compartmental model may be selected: for example, apparent k4>0 in FDG studies may be caused by tissue heterogeneity, not by dephosphorylation of FDG-6-phosphate (Schmidt et al., 1992). In the commonly used two-tissue compartmental model the two settings, where tissue compartments are in series or in parallel, are kinetically indistinguishable from each other.
Results from multiple-time graphical analysis (MTGA) and FUR represent (weighed) average of tissues inside the VOI. Tissue heterogeneity does not cause any bias.
Also regional SUV represents the non-biased average SUV of tissues inside the VOI. However, the optimal scan time for SUV calculation may change, and thus affect the results.
Measurement of tissue heterogeneity
Stochastic dynamic model has been proposed as one possibility to estimate mean compartmental model rate constants and their variance (Niemi et al., 2007). Considering also the image reconstruction error may further improve the tissue heterogeneity estimation (Forma et al., 2013).
Fractal analysis (FA) has been used in several PET and SPET studies to quantitate the distribution of blood flow in tissues (Kuikka et al., 1997; Venegas & Galletti, 2000; Nagao et al., 2001; Kalliokoski et al., 2001a; and 2003b). FA has also been used to assess regional heterogeneity in tumours, for instance in PSMA (Sachpekidis et al., 2020) and L-[methyl-11C]methionine (Maeda et al., 2021) PET imaging.
The simplest method to quantitate heterogeneity is the calculation of variance of voxel values inside a VOI drawn inside an organ. This method is naturally affected by all factors that affect the PET image quality, but it still provides meaningful results as long as the study protocol is exactly same for the whole study population. This method has been applied to the skeletal muscle (Kalliokoski et al., 2004; Laaksonen et al., 2010).
Tissue heterogeneity and PVE lead to biases in results of compartmental models which assume tissue homogeneity. Especially K1/k2 tends to be biased (underestimated), which is apparent in the partition coefficient in [15O]H2O brain studies.
See also:
References:
Asselin M-C, O'Connor JPB, Boellaard R, Thacker NA, Jackson A. Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer 2012; 48: 447-455. doi: 10.1016/j.ejca.2011.12.025.
Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res. 1989; 65:578-590. PMID: 2766485.
Blomqvist G, Lammertsma AA, Mazoyer B, Wienhard K. Effect of tissue heterogeneity on quantification in positron emission tomography. Eur J Nucl Med. 1995; 22: 652-663. doi: 10.1007/BF01254567.
Brooks FJ. On some misconceptions about tumor heterogeneity quantification. Eur J Nucl Med Mol Imaging 2013; 40: 1292-1294. doi: 10.1007/s00259-013-2430-y.
Brooks FJ, Grigsby PW. The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med. 2014; 55: 37-42. doi: 10.2967/jnumed.112.116715.
Cheng N-M, Fang Y-HD, Yen T-C. The promise and limits of PET texture analysis. Ann Nucl Med. 2013; 27: 867-869. doi: 10.1007/s12149-013-0759-8.
Eary JF, O'Sullivan F, O'Sullivan J, Conrad EU. Spatial heterogeneity in sarcoma 18F-FDG uptake as a predictor of patient outcome. J Nucl Med. 2008; 49(12): 1973-1979. doi: 10.2967/jnumed.108.053397.
Forma J, Niemi JA, Ruotsalainen U. Regional compensation for statistical maximum likelihood reconstruction error of PET image pixels. Phys Med Biol. 2013; 58: 4849-4864. doi: 10.1088/0031-9155/58/14/4849.
Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol. 2007; 103: 2042-2048. doi: 10.1152/japplphysiol.00567.2007.
Herholz K, Patlak CS. The influence of tissue heterogeneity on results of fitting nonlinear model equations to regional tracer uptake curves. With an application to compartmental models used in positron emission tomography. J Cereb Blood Flow Metab. 1987; 7: 214-229. doi: 10.1038/jcbfm.1987.47.
Kalliokoski KK, Kemppainen J, Larmola K, Takala TO, Peltoniemi P, Oksanen A, Ruotsalainen U, Cobelli C, Knuuti J, Nuutila P. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur J Appl Physiol. 2000; 83: 395-401. doi: 10.1007/s004210000267.
Kalliokoski KK, Kuusela TA, Nuutila P, Tolvanen T, Oikonen V, Teräs M, Takala TE, Knuuti J. Perfusion heterogeneity in human skeletal muscle: fractal analysis of PET data. Eur J Nucl Med. 2001a; 28: 450-456. doi: 10.1007/s002590000458.
Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001b; 280: E1015-E1021. doi: 10.1152/ajpendo.2001.280.6.E1015.
Kalliokoski KK, Laaksonen MS, Takala TO, Knuuti J, Nuutila P. Muscle oxygen extraction and perfusion heterogeneity during continuous and intermittent static exercise. J Appl Physiol. 2003a; 94: 953-958. doi: 10.1152/japplphysiol.00731.2002.
Kalliokoski KK, Kuusela TA, Laaksonen MS, Knuuti J, Nuutila P. Muscle fractal vascular branching pattern and microvascular perfusion heterogeneity in endurance-trained and untrained men. J Physiol. 2003b; 546: 529-535. doi: 10.1113/jphysiol.2002.030882.
Kalliokoski KK, Knuuti J, Nuutila P. Blood transit time heterogeneity is associated to oxygen extraction in exercising human skeletal muscle. Microvasc Res. 2004; 67: 125-132. doi: 10.1016/j.mvr.2003.11.004.
Kuikka JT. Effect of tissue heterogeneity on quantification in positron emission tomography. Eur J Nucl Med. 1995; 22: 1457-1458. doi: 10.1007/BF01791155.
Kuikka JT, Tiihonen J, Karhu J. Fractal analysis of striatal dopamine re-uptake sites. Eur J Nucl Med. 1997; 24: 1085-1090. doi: 10.1007/BF01254238.
Laaksonen MS, Björklund G, Heinonen I, Kemppainen J, Knuuti J, Kyröläinen H, Kalliokoski KK. Perfusion heterogeneity does not explain excess muscle oxygen uptake during variable intensity exercise. Clin Physiol Funct Imaging 2010; 30: 241-249. doi: 10.1111/j.1475-097X.2010.00934.x.
Lammertsma AA, Jones T. Low oxygen extraction in tumours measured with the oxygen-15 steady state technique: effect of tissue heterogeneity. Br J Radiol. 1992; 65: 697-700. doi: 10.1259/0007-1285-65-776-697.
Nagao M, Murase K, Kikuchi T, Ikeda M, Nebu A, Fukuhara R, Sugawara Y, Miki H, Ikezoe J. Fractal analysis of cerebral blood flow distribution in Alzheimer's disease. J Nucl Med. 2001; 42: 1446-1450. PMID: 11585855.
Niemi J, Ruotsalainen U, Saarinen A, Ruohonen K. Stochastic dynamic model for estimation of rate constants and their variances from noisy and heterogeneous PET measurements. Bull Math Biol. 2007; 69: 585-604. doi: 10.1007/s11538-006-9150-4.
O'Sullivan F, Roy S, Eary J. A statistical measure of tissue heterogeneity with application to 3D PET sarcoma data. Biostatistics 2003; 4(3): 433-448. doi: 10.1093/biostatistics/4.3.433.
Schmidt K, Lucignani G, Moresco RM, Rizzo G, Gilardi MC, Messa C, Colombo F, Fazio F, Sokoloff L. Errors introduced by tissue heterogeneity in estimation of local cerebral glucose utilization with current kinetic models of the [18F]fluorodeoxyglucose method. J Cereb Blood Flow Metab. 1992; 12: 823-834. doi: 10.1038/jcbfm.1992.114.
Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med. 2012; 53: 693-700. doi: 10.2967/jnumed.111.099127.
Vriens D, Disselhorst JA, Oyen WJG, de Geus-Oei L-F, Visser EP. Quantitative assessment of heterogeneity in tumor metabolism using FDG-PET. Int J Radiation Oncol Biol Phys. 2012; 82(5): e725-e731. doi: 10.1016/j.ijrobp.2011.11.039.
Willaime JMY, Turkheimer FE, Kenny LM, Aboagye EO. Quantification of intra-tumour cell proliferation heterogeneity using imaging descriptors of 18F fluorothymidine-positron emission tomography. Phys Med Biol. 2013; 58: 187-203. doi: 10.1088/0031-9155/58/2/187.
Tags: Heterogeneity, PVE, Fractal analysis
Updated at: 2021-12-22
Created at: 2014-05-07
Written by: Vesa Oikonen