First-order kinetics
Dynamic processes in vivo
In living body, we are observing dynamic biochemical processes, which include:
1. Translocation
Translocation is the class of processes that results in movement of a chemical species, for example radioligand molecule, from one location to another.
|
|
2. Transformation
Transformation is the class of processes that results in conversion of one molecular species to another (biochemical reactions).
|
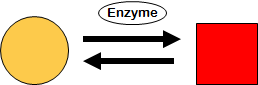
|
3. Binding
Binding is the class of processes that consist of two molecular species combining, usually non-covalently, to form a single complex. Target is the macromolecular participant in this process. Binding is actually a special case of transformation, but as a widespread biological phenomenon it deserves a separate category.
|

|
Specific binding
Specific binding is defined as the radioligand binding to the target molecule, not including the binding to other macromolecular components or radioligand that is not bound at all (free) in the tissue sample.
First-order kinetics
Dynamic process is of "first-order", when its speed depends on one concentration only. Standard mathematical methods in PET modelling assume first-order kinetics.
For a first-order process A➝P, the velocity v can be expressed as
, where k is a first-order rate constant: k is independent of concentration of substrate A (CA) and time (t); its unit is sec-1 or min-1.
First-order kinetics means that the rate of the dynamic process can be represented by a single rate constant, first-order rate constant, k. This k is constant, it does not change if concentration of substrate A or product P changes. Lets say that k is 0.1 in units 1/minute; that means that 10% of A will be processed into P in one minute. When all substrate is processed into product, reaction velocity is 0, but k is still 0.1.
Note that 1.0 is not an upper limit for a rate constant!
Compartmental models can be described with exponential functions. The concentration of the substrate A at time t can be calculated from equation
Differentiating this equation will give:
Pseudo-first-order kinetics
Dynamic biochemical processes always involve two or more reactants. If we keep the concentration of one reactant very small compared to the others, equations simplify to the same form as for first-order kinetics.
When we inject the radioligand in tracer dose (very small mass), the rate constant will be independent on the concentration of the radioligand itself. Then the rate constant k is only dependent on the other reactant, for example, activity and concentration of an enzyme, or concentration and affinity of receptor, or concentration and affinity of transporter protein.
Dynamic process is evaluated in a steady state, or dynamic equilibrium:
- Rate of process is not changing with time
- Amount of tracee is constant during the evaluation period
- Steady state of the tracer is not required.
Only when these requirements are satisfied, the processes can be described with first-order rate constants.
See also:
- Tracer
- PET data
- Input function
- Compartmental models
- Fitting compartmental models
- Plasma pharmacokinetics
- Derivative Calculator
Tags: Modeling, Rate constant, Exponential function
Updated at: 2019-01-13
Created at: 2006-08-08
Written by: Vesa Oikonen