Partial volume effect and correction
Partial volume effect (PVE) is a combination of two factors, the limited resolution of PET, and image sampling (Hoffman et al., 1979; Kessler et al., 1984). Image sampling refers to the fact that PET voxel has a definite volume, which may consist only partially of the desired tissue, reflecting underlying tissue heterogeneity (Aston et al., 2002). Together these factors blur the images. Multiple tissue types contribute to the measured radioactivity concentration of even single voxels, and more so to the volumes-of-interest (VOI) consisting of many voxels.
Typically PVE is seen in tumour and brain imaging as spill-out of radioactivity into surrounding tissue from a high-activity region (tumour, brain cortex), leading to underestimation of tracer uptake estimates (Figure 1). It can also be seen as spill-in (spill-over) into VOI from high-activity region (heart cavities, large arteries and veins, urine), leading to overestimation of radiotracer uptake estimates in for example myocardial muscle and bladder wall (Figure 2). Typically both spill-out and spill-in effects are present (Figure 3). Defluorination of 18F-labelled radiopharmaceuticals can lead to high uptake in skull bone, preventing accurate quantification of cortical activity. PVE is also the major hindrance in extracting blood curve from arteries that are visible in PET image.
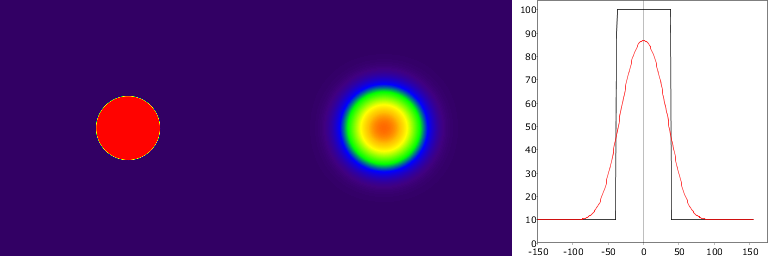
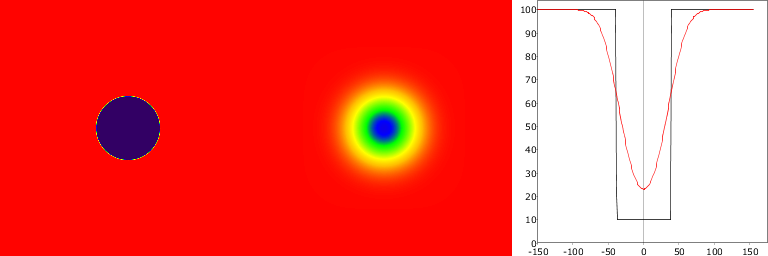

These demonstration were make with script that is available in GitLab.
Partial volume correction
Partial volume correction (PVC) becomes important when the object size is less than
two times the spatial resolution (FWHM) in the image.
Correction may be based on empiric recovery coefficients (RCs)
(Geworski et al., 2000).
More accurate correction is possible if the point-spread function (PSF) of the tomograph
is known. Addressing the tissue-fraction effect requires coregistered high-resolution
MR images
(Rousset et al., 1998).
The partial volume correction is studied in detail by
Aston et al (2002), and methods are
reviewed by Rousset et al (2007),
Soret et al (2007),
Erlandsson et al (2012), and
Bettinardi et al (2014).
One of the international software projects is PVEOut.
PETPVC is another open-source package for partial volume
correction (Thomas et al., 2016).
Alternative methods have been implemented, including accounting for PSF in the
image reconstruction process; however, current PSF
reconstruction may lead to serious image artifacts
(Munk et al., 2017).
Model-free spill-over correction of PET images based on iterative deconvolution method
(IDM),
requiring only image resolution (FWHM or PSF) has performed well in comparison to anatomical image
based methods (Tohka & Reilhac, 2006
and 2008;
Teo et al., 2007;
Golla et al., 2017;
Cysouw et al., 2019).
Partial volume correction, denoising, and image segmentation are dependent on each other, and
a joint solution can provide better results than performing each step separately
(Xu et al., 2018).
There are several studies on the impact of PVE on clinical PET results, especially in oncology and brain research. For example, in [18F]FDOPA studies PVE leads to severe underestimation of Ki and k3D in certain brain structures, thus obscuring regional heterogeneity in the neurochemical pathology of Parkinson's disease (Rousset et al 2000). In the early studies, PVC was accomplished by fitting image profiles (Yu et al., 1993).
In brain PET studies, K1/k2 in regions of interest is often fixed to a value estimated first in the reference region. Because PVE is different between brain structures, this may lead into biases. For example, PVC increased the K1/k2 of cerebral cortex by 35 % in [18F]FDOPA studies (Rousset et al., 2000). The bias in K1/k2 is very apparent in [15O]H2O brain studies. However, measured receptor occupancy is independent of partial volume effect (Martinez et al., 2001).
Proper PVC is of critical importance in accurate quantitative PET, especially in ageing studies of the brain, where the apparent reduction in metabolic activity of cortex is disappeared after PVC (Giovacchini et al., 2004).
Spill-out from skull, caused by defluorination of the radiopharmaceutical, can sometimes be taken into account in the model (Tsartsalis et al., 2014).
Similar error sources
Different attenuation correction methods may result in apparent changes in local radioactivity concentrations, which may be misinterpreted as PVE. For instance, using CT-based attenuation correction may produce higher activities than Germanium source based attenuation correction, especially in radiodense tissue like bone (Nakamoto et al., 2002). Patient movement between transmission measurement and PET scan may lead to spurious results. Imaging of trunk region is affected by respiratory and cardiac motion; image blurring can be minimized with gating.
See also:
- Partial volume and spillover effects in cardiac PET
- PVE in brain [15O]H2O PET
- Tissue heterogeneity
- Volume of interest
- Image-derived input function
- Image filtering
- Factor analysis
- Image clustering
Literature
Akamatsu G, Shimada N, Matsumoto K, Daisaki H, Suzuki K, Watabe H, Oda K, Senda M, Terauchi T, Tateishi U. New standards for phantom image quality and SUV harmonization range for multicenter oncology PET studies. Ann Nucl Med. 2022; 36(2): 144-161. doi: 10.1007/s12149-021-01709-1.
Aston JAD, Cunningham VJ, Asselin M-C, Hammers A, Evans AC, Gunn RN. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab. 2002; 22: 1019-1034. doi: 10.1097/00004647-200208000-00014.
Bowen SL, Byars LG, Michel CJ, Chonde DB, Catana C. Influence of the partial volume correction method on 18F-fluorodeoxyglucose brain kinetic modelling from dynamic PET images reconstructed with resolution model based OSEM. Phys Med Biol. 2013; 58: 7081-7106. doi: 10.1088/0031-9155/58/20/7081.
Erlandsson K, Buvat I, Pretorius PH, Thomas BA, Hutton BF. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys Med Biol. 2012; 57: R119-R159 doi: 10.1088/0031-9155/57/21/R119.
Erlandsson K, Dickson J, Arridge S, Atkinson D, Ourselin S, Hutton BF. MR imaging-guided partial volume correction of PET data in PET/MR imaging. PET Clin. 2016; 11: 161-177. doi: 10.1016/j.cpet.2015.09.002.
Funck T, Larcher K, Toussaint PJ, Evans AC, Thiel A. APPIAN: Automated Pipeline for PET Image Analysis. Front Neuroinform. 2018; 12: 64. doi: 10.3389/fninf.2018.00064.
Geworski L, Knoop BO, de Cabrejas ML, Knapp WH, Munz DL. Recovery correction for quantitation in emission tomography: a feasibility study. Eur J Nucl Med. 2000; 27(2): 161-169. doi: 10.1007/s002590050022.
Gigengack F, Jiang X, Dawood M, Schäfers KP: Motion Correction in Thoracic Positron Emission Tomography. Springer, 2015. doi: 10.1007/978-3-319-08392-6.
Golla SVS, Lubberink M, van Berckel BNM, Lammertsma AA, Boellaard R. Partial volume correction of brain PET studies using iterative deconvolution in combination with HYPR denoising. EJNMMI Res. 2017; 7: 36. doi: 10.1186/s13550-017-0284-1.
Hofheinz F, Langner J, Petr J, Beuthien-Baumann B, Oehme L, Steinbach J, Kotzerke J, van den Hoff J. A method for model-free partial volume correction in oncological PET. EJNMMI Res. 2012; 2:16. doi: 10.1186/2191-219X-2-16.
Jomaa H, Mabrouk R, Khlifa N. Post-reconstruction-based partial volume correction methods: a comprehensive review. Biomed Signal Proces. 2018; 46: 131-144. doi: 10.1016/j.bspc.2018.05.029.
Kessler RM, Ellis JR Jr, Eden M. Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr. 1984; 8(3): 514-522. PMID: 6609942.
Merisaari H. Algorithmic analysis techniques for molecular imaging. Turku Centre for Computer Science, TUCS Dissertations, 217, 2016.
Munk OL, Tolbod LP, Hansen SB, Bogsrud TV. Point-spread function reconstructed PET images of sub-centimeter lesions are not quantitative. EJNMMI Physics 2017; 4:5. doi: 10.1186/s40658-016-0169-9.
Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998; 39(5): 904-911. PMID: 9591599.
Thomas BS, Cuplov V, Bousse A, Mendes A, Thielemans K, Hutton BF, Erlandsson K. PETPVC: a toolbox for performing partial volume correction techniques in positron emission tomography. Phys med Biol. 2016; 61: 7975-7993. doi: 10.1088/0031-9155/61/22/7975.
Tohka J, Reilhac A. Deconvolution-based partial volume correction in Raclopride-PET and Monte Carlo comparison to MR-based method. NeuroImage 2008; 39: 1570-1584. doi: 10.1016/j.neuroimage.2007.10.038.
Tags: Image, PVE, Heterogeneity
Updated at: 2019-11-22
Created at: 2004-09-09
Written by: Vesa Oikonen