Muscle [15O]H2O PET
Radiowater bolus-injection, or [15O]CO2 inhalation studies can be analyzed with kinetic model fitting or autoradiographic (ARG) method. Both methods are based on the same model for [15O]H2O, and both can be applied to dynamic PET scan data, but static PET image data can only be analyzed with the ARG method.
Steady-state [15O]H2O or [15O]CO2 studies of skeletal muscle (Ruotsalainen et al., 1997) are not commonly performed on humans due to relatively high radiation dose and duration of the scan, but they are applicable for in animal studies (Carter et al., 1997).
Arterial blood data, collected using on-line sampling system, must be calibrated and corrected for physical decay, dispersion and time delay. Time delay may not be the same in different legs or muscle regions, and therefore it should be corrected for each region separately; when computing parametric images this is not possible for statistical reasons (Burchert et al., 1997).
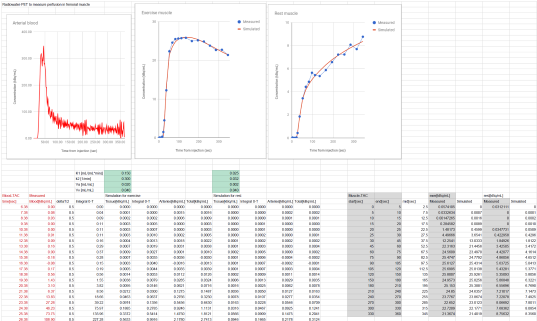
One-tissue compartmental model can be used to simulate realistic muscle PET time-radioactivity concentration curves even in spreadsheet. Download the spreadsheet in Google Docs, Excel (.xlsx), or OpenDocument (.ods) format.
Exercise studies, when one leg is at rest, can also be analyzed semi-quantitatively by calculating exercise-to-resting muscle ROI ratios (Ament et al., 1998); radioactivity concentration has roughly linear relationship with muscle perfusion (Burchert et al., 1997).
Parametric perfusion images
ARG method
ARG method can be used to calculate the perfusion in skeletal muscle (Ruotsalainen et al., 1997). Method can produce parametric blood flow images in good quality, and results are reliable, because partition volume of water in skeletal muscle is relatively constant and arterial volume fraction is usually low and relatively stable.
In ARG method, a fixed partition coefficient (p) and arterial blood volume (VA) must be used. For skeletal muscle usual assumptions are p=0.99 and VA=0.
Kinetic method
If dynamic PET data is collected, then basis function (Boellaard et al., 2005) and multilinear approach (linearization described by van den Hoff et al., 1993, and applied to skeletal muscle by Burchert et al., 1997) for kinetic model are viable alternatives to the ARG method, and may provide better quantification especially if there are large perfusion differences in the regions of interest, or arterial volume fraction is not small or can vary greatly, or if also other tissues (with different or unknown partition coefficient of water) than skeletal muscle are to be analyzed from the same PET image. In practice, partition coefficient may be impossible to measure accurately in resting muscle where perfusion is low (Burchert et al., 1997).
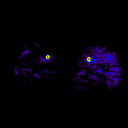

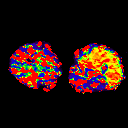
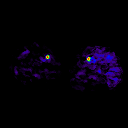
Fischman et al (2002) validated kinetic analysis of [15O]H2O PET in dog hindlimb muscles against microsphere method in a wide perfusion range.
Clinical results
Peripheral vascular disease (PVD) has been studied with radiowater PET, but no significant differences have been found between patients and healthy subjects (Depairon et al., 1991; Burchert et al., 1997). Exercise-induced perfusion increase was significantly lower in ischemic than in normal legs, which can help to identify severely ischemic leg regions and to decide the level of amputation (Scremin et al, 2010).
Literature
Ament W, Lubbers J, Rakhorst G, Vaalburg W, Verkerke GJ, Paans AM, Willemsen AT. Skeletal muscle perfusion measured by positron emission tomography during exercise. Pflugers Arch. 1998; 436(5): 653-658. doi: 10.1007/s004240050685.
Barrett EJ, Rattigan S. Muscle perfusion: its measurement and role in metabolic regulation. Diabetes 2012; 61(11): 2661-2668. doi: 10.2337/db12-0271.
Boellaard R, Knaapen P, Rijbroek A, Luurtsema GJ, Lammertsma AA. Evaluation of basis function and linear least squares methods for generating parametric blood flow images using 15O-water and positron emission tomography. Mol Imaging Biol. 2005; 7(4): 273-285. doi: 10.1007/s11307-005-0007-2.
Burchert W, Schellong S, van den Hoff J, Meyer GJ, Alexander K, Hundeshagen H. Oxygen-15-water PET assessment of muscular blood flow in peripheral vascular disease. J Nucl Med. 1997; 38(1): 93-98.
Carter EA, Tompkins RG, Hsu H, Christian B, Alpert NM, Weise S, Fischman AJ. Metabolic alterations in muscle of thermally injured rabbits, measured by positron emission tomography. Life Sci. 1997; 61(1): 39-44. doi: 10.1016/S0024-3205(97)00355-X.
Depairon M, De Landsheere C, Merlo P, Del Fiore G, Quaglia L, Peters JM, Lamotte D, Zicot M. Effect of exercise on the leg distribution of C15O2 and 15O2 in normals and in patients with peripheral ischemia: a study using positron tomography. Int Angiol. 1988; 7(3): 254-257.
Depairon M, Depresseux JC, Petermans J, Zicot M. Assessment of flow and oxygen delivery to the lower extremity in arterial insufficiency: a PET-scan study comparison with other methods. Angiology 1991; 42(10): 788-795. doi: 10.1177/000331979104201003.
Depairon M, Zicot M. The quantitation of blood flow/metabolism coupling at rest and after exercise in peripheral arterial insufficiency, using PET and 15-O labeled tracers. Angiology 1996; 47(10): 991-999. doi: 10.1177/000331979604701008.
Fischman AJ, Hsu H, Carter EA, Yu YM, Tompkins RG, Guerrero JL, Young VR, Alpert NM. Regional measurement of canine skeletal muscle blood flow by positron emission tomography with H215O. J Appl Physiol. 2002; 92(4): 1709-1716. doi: 10.1152/japplphysiol.00445.2001.
Hannukainen JC, Nuutila P, Kaprio J, Heinonen OJ, Kujala UM, Janatuinen T, Rönnemaa T, Kapanen J, Haaparanta-Solin M, Viljanen T, Knuuti J, Kalliokoski KK. Relationship between local perfusion and FFA uptake in human skeletal muscle - no effect of increased physical activity and aerobic fitness. J Appl Physiol. 2006; 101(5): 1303-1311. doi: 10.1152/japplphysiol.00012.2006.
Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol. 2007; 103(6): 2042-2048. doi: 10.1152/japplphysiol.00567.2007.
Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, Oikonen V, Nuutila P, Knuuti J, Hellsten Y, Boushel R, Kalliokoski KK. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol. 2010; 108(2): 378-386. doi: 10.1152/japplphysiol.00745.2009.
Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol. 2011; 111(3): 818-824. doi: 10.1152/japplphysiol.00269.2011.
Heinonen I, Saltin B, Hellsten Y, Kalliokoski KK. The effect of nitric oxide synthase inhibition with and without inhibition of prostaglandins on blood flow in different human skeletal muscles. Eur J Appl Physiol. 2017; 117(6): 1175-1180. doi: 10.1007/s00421-017-3604-2.
Hällsten K, Yki-Järvinen H, Peltoniemi P, Oikonen V, Takala T, Kemppainen J, Laine H, Bergman J, Bolli GB, Knuuti J, Nuutila P. Insulin- and exercise-stimulated skeletal muscle blood flow and glucose uptake in obese men. Obes Res. 2003; 11(2): 257-265. doi: 10.1038/oby.2003.39.
Kalliokoski KK, Kemppainen J, Larmola K, Takala TO, Peltoniemi P, Oksanen A, Ruotsalainen U, Cobelli C, Knuuti J, Nuutila P. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur J Appl Physiol. 2000; 83(4 -5): 395-401. doi: 10.1007/s004210000267.
Kalliokoski KK, Laaksonen MS, Knuuti J, Nuutila P. Perfusion distribution between and within muscles during intermittent static exercise in endurance-trained and untrained men. Int J Sports Med. 2003; 24(6): 400-403. doi: 10.1055/s-2003-41179.
Kalliokoski KK, Langberg H, Ryberg AK, Scheede-Bergdahl C, Doessing S, Kjaer A, Kjaer M, Boushel R. Nitric oxide and prostaglandins influence local skeletal muscle blood flow during exercise in humans: coupling between local substrate uptake and blood flow. Am J Physiol Regul Integr Comp Physiol. 2006; 291(3): R803-R809. doi: 10.1152/ajpregu.00808.2005.
Keenan GF, Ashcroft GP, Roditi GH, Hutchison JD, Evans NT, Mikecz P, Chaloner F, Dodd M, Leonard C, Porter RW, et al. Measurement of lower limb blood flow in patients with neurogenic claudication using positron emission tomography. Spine; 1995; 20(4): 408-411. doi: 10.1097/00007632-199502001-00002.
Laaksonen MS, Kalliokoski KK, Kyröläinen H, Kemppainen J, Teräs M, Sipilä H, Nuutila P, Knuuti J. Skeletal muscle blood flow and flow heterogeneity during dynamic and isometric exercise in humans. Am J Physiol Heart Circ Physiol. 2003; 284(3): H979-H986. doi: 10.1152/ajpheart.00714.2002.
Laaksonen MS, Kemppainen J, Kyröläinen H, Knuuti J, Nuutila P, Kalliokoski KK. Regional differences in blood flow, glucose uptake and fatty acid uptake within quadriceps femoris muscle during dynamic knee-extension exercise. Eur J Appl Physiol. 2013; 113(7): 1775-1782. doi: 10.1007/s00421-013-2609-8.
Laine H, Knuuti MJ, Ruotsalainen U, Raitakari M, Iida H, Kapanen J, Kirvelä O, Haaparanta M, Yki-Järvinen H, Nuutila P. Insulin resistance in essential hypertension is characterized by impaired insulin stimulation of blood flow in skeletal muscle. J Hypertens. 1998; 16(2): 211-219. PMID: 9535149.
Laine H, Yki-Jarvinen H, Kirvela O, Tolvanen T, Raitakari M, Solin O, Haaparanta M, Knuuti J, Nuutila P. Insulin resistance of glucose uptake in skeletal muscle cannot be ameliorated by enhancing endothelium-dependent blood flow in obesity. J Clin Invest. 1998; 101(5): 1156-1162. doi: 10.1172/JCI1065.
Laine H, Knuuti MJ, Ruotsalainen U, Utriainen T, Oikonen V, Raitakari M, Luotolahti M, Kirvelä O, Vicini P, Cobelli C, Nuutila P, Yki-Järvinen H. Preserved relative dispersion but blunted stimulation of mean flow, absolute dispersion, and blood volume by insulin in skeletal muscle of patients with essential hypertension. Circulation 1998; 97(21): 2146-2153. doi: 10.1161/01.cir.97.21.2146.
Lodge MA, Carson RE, Carrasquillo JA, Whatley M, Libutti SK, Bacharach SL. Parametric images of blood flow in oncology PET studies using [15O]water. J Nucl Med 2000; 41:1784-1792. PMID: 11079484.
Malminiemi K, Laine H, Knuuti MJ, Ruotsalainen U, Oikonen V, Haaparanta M, Nuutila P. Acute effects of celiprolol on muscle blood flow and insulin sensitivity: studies using [15O]-water, [18F]-fluorodeoxyglucose and positron emission tomography. Eur J Clin Pharmacol. 1997; 52(1): 19-26. doi: 10.1007/s002280050243.
Matic DB, Lee TY, Wells RG, Gan BS. The effects of botulinum toxin type A on muscle blood perfusion and metabolism. Plast Reconstr Surg. 2007; 120(7): 1823-1833. doi: 10.1097/01.prs.0000287135.17291.2f.
Mizuno M, Kimura Y, Iwakawa T, Oda K, Ishii K, Ishiwata K, Nakamura Y, Muraoka I. Regional differences in blood volume and blood transit time in resting skeletal muscle. Jpn J Physiol. 2003; 53(6): 467-470. doi: 10.2170/jjphysiol.53.467.
Mizuno M, Kimura Y, Iwakawa T, Oda K, Ishii K, Ishiwata K, Nakamura Y, Muraoka I. Regional differences in blood flow and oxygen consumption in resting muscle and their relationship during recovery from exhaustive exercise. J Appl Physiol. 2003; 95(6): 2204-2210. doi: 10.1152/japplphysiol.00197.2003.
Mossberg KA, Mullani N, Gould KL, Taegtmeyer H. Skeletal muscle blood flow in vivo: detection with rubidium-82 and effects of glucose, insulin, and exercise. J Nucl Med. 1987; 28(7): 1155-1163. PMID: 3298572.
Nuutila P, Raitakari M, Laine H, Kirvelä O, Takala T, Utriainen T, Mäkimattila S, Pitkänen OP, Ruotsalainen U, Iida H, Knuuti J, Yki-Järvinen H. Role of blood flow in regulating insulin-stimulated glucose uptake in humans. Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest. 1996; 97(7): 1741-1747. doi: 10.1172/JCI118601.
Nuutila P, Kalliokoski K. Use of positron emission tomography in the assessment of skeletal muscle and tendon metabolism and perfusion. Scand J Med Sci Sports 2000; 10(6): 346-350. doi: 10.1034/j.1600-0838.2000.010006346.x.
Nuutila P, Peltoniemi P, Oikonen V, Larmola K, Kemppainen J, Takala T, Sipilä H, Oksanen A, Ruotsalainen U, Bolli GB, Yki-Järvinen H. Enhanced stimulation of glucose uptake by insulin increases exercise-stimulated glucose uptake in skeletal muscle in humans: studies using [15O]O2, [15O]H2O, [18F]fluoro-deoxy-glucose, and positron emission tomography. Diabetes 2000; 49:1084-1091. doi: 10.2337/diabetes.49.7.1084.
Orbay H, Hong H, Zhang Y, Cai W. PET/SPECT imaging of hindlimb ischemia: focusing on angiogenesis and blood flow. Angiogenesis 2013; 16(2): 279-287. doi: 10.1007/s10456-012-9319-4.
Paternostro G, Camici PG, Lammerstma AA, Marinho N, Baliga RR, Kooner JS, Radda GK, Ferrannini E. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest. 1996; 98(9): 2094-2099. doi: 10.1172/JCI119015.
Pitkänen OP, Laine H, Kemppainen J, Eronen E, Alanen A, Raitakari M, Kirvelä O, Ruotsalainen U, Knuuti J, Koivisto VA, Nuutila P. Sodium nitroprusside increases human skeletal muscle blood flow, but does not change flow distribution or glucose uptake. J Physiol. 1999; 521(Pt 3): 729-737. doi: 10.1111/j.1469-7793.1999.00729.x.
Raichle ME. Quantitative in vivo autoradiography with positron emission tomography. Brain Res Rev. 1979; 1: 47-68. doi: 10.1016/0165-0173(79)90016-X.
Raitakari M, Knuuti MJ, Ruotsalainen U, Laine H, Mäkelä P, Teräs M, Sipilä H, Niskanen T, Raitakari OT, Iida H, Härkönen R, Wegelius U, Yki-Järvinen H, Nuutila P. Insulin increases blood volume in human skeletal muscle: studies using [15O]CO and positron emission tomography. Am J Physiol. 1995; 269(6 Pt 1): E1000-E1005. doi: 10.1152/ajpendo.1995.269.6.E1000.
Raitakari M, Nuutila P, Ruotsalainen U, Teräs M, Eronen E, Laine H, Raitakari OT, Iida H, Knuuti MJ, Yki-Järvinen H. Relationship between limb and muscle blood flow in man. J Physiol. 1996; 496(Pt 2): 543-549. doi: 10.1113/jphysiol.1996.sp021705.
Raitakari M, Nuutila P, Ruotsalainen U, Laine H, Teräs M, Iida H, Mäkimattila S, Utriainen T, Oikonen V, Sipilä H, Haaparanta M, Solin O, Wegelius U, Knuuti J, Yki-Järvinen H. Evidence for dissociation of insulin stimulation of blood flow and glucose uptake in human skeletal muscle: studies using [15O]H2O, [18F]fluoro-2-deoxy-D-glucose, and positron emission tomography. Diabetes 1996; 45(11): 1471-1477. doi: 10.2337/diab.45.11.1471.
Raitakari M, Nuutila P, Knuuti J, Raitakari OT, Laine H, Ruotsalainen U, Kirvelä O, Takala TO, Iida H, Yki-Järvinen H. Effects of insulin on blood flow and volume in skeletal muscle of patients with IDDM: studies using [15O]H2O, [15O]CO, and positron emission tomography. Diabetes 1997; 46(12): 2017-2021. doi: 10.2337/diab.46.12.2017.
Rudroff T, Weissman JA, Bucci M, Seppänen M, Kaskinoro K, Heinonen I, Kalliokoski KK. Positron emission tomography detects greater blood flow and less blood flow heterogeneity in the exercising skeletal muscles of old compared with young men during fatiguing contractions. J Physiol. 2014; 592(2): 337-349. doi: 10.1113/jphysiol.2013.264614.
Ruotsalainen U, Raitakari M, Nuutila P, Oikonen V, Sipilä H, Teräs M, Knuuti J, Bloomfield PM, Iida H. Quantitative blood flow measurement of skeletal muscle using oxygen-15-water and PET. J Nucl Med. 1997; 38: 314-319.
Rådegran G. Limb and skeletal muscle blood flow measurements at rest and during exercise in human subjects. Proc Nutr Soc. 1999; 58(4): 887-898. PMID: 10817156.
Rönnemaa EM, Rönnemaa T, Utriainen T, Raitakari M, Laine H, Takala T, Pitkänen OP, Kirvelä O, Knuuti J, Nuutila P. Decreased blood flow but unaltered insulin sensitivity of glucose uptake in skeletal muscle of chronic smokers. Metabolism 1999; 48(2): 239-244. doi: 10.1016/S0026-0495(99)90041-0.
Schellong SM, Böger RH, Burchert W, Bode-Böger SM, Galland A, Frölich JC, Hundeshagen H, Alexander K. Dose-related effect of intravenous L-arginine on muscular blood flow of the calf in patients with peripheral vascular disease: a H215O positron emission tomography study. Clin Sci (Lond) 1997; 93(2): 159-165. doi: 10.1042/cs0930159.
Schellong SM, Burchert W, Böger RM, Creutzig A, Hundeshagen H, Alexander K. Prostaglandin E1 in peripheral vascular disease: a PET study of muscular blood flow. Scand J Clin Lab Invest. 1998; 58(2): 109-117. doi: 10.1080/00365519850186689.
Schmidt MA, Chakrabarti A, Shamim-Uzzaman Q, Kaciroti N, Koeppe RA, Rajagopalan S. Calf flow reserve with H215O PET as a quantifiable index of lower extremity flow. J Nucl Med. 2003; 44(6): 915-919. PMID: 12791819.
Scremin OU, Cuevas-Trisan RL, Scremin AM, Brown CV, Mandelkern MA. Functional electrical stimulation effect on skeletal muscle blood flow measured with H215O positron emission tomography. Arch Phys Med Rehabil. 1998; 79(6): 641-646. doi: 10.1016/S0003-9993(98)90037-5.
Scremin OU, Figoni SF, Norman K, Scremin AM, Kunkel CF, Opava-Rutter D, Schmitter ED, Bert A, Mandelkern M. Preamputation evaluation of lower-limb skeletal muscle perfusion with H215O positron emission tomography. Am J Phys Med Rehabil. 2010; 89(6): 473-486. doi: 10.1097/PHM.0b013e3181d89b08.
Stacy MR, Zhou W, Sinusas AJ. Radiotracer imaging of peripheral vascular disease. J Nucl Med Technol. 2015; 43(3): 185-292. doi: 10.2967/jnumed.112.115105.
Utriainen T, Nuutila P, Takala T, Vicini P, Ruotsalainen U, Rönnemaa T, Tolvanen T, Raitakari M, Haaparanta M, Kirvelä O, Cobelli C, Yki-Järvinen H. Intact insulin stimulation of skeletal muscle blood flow, its heterogeneity and redistribution, but not of glucose uptake in non-insulin-dependent diabetes mellitus. J Clin Invest. 1997; 100(4): 777-785. doi: 10.1172/JCI119591.
van den Hoff J, Burchert W, Müller-Schauenburg W, Meyer G-J, Hundeshagen H. Accurate local blood flow measurements with dynamic PET: fast determination of input function delay and dispersion by multilinear minimization. J Nucl Med. 1993; 34: 1770-1777. PMID: 8410297.
Vanderthommen M, Depresseux JC, Bauvir P, Degueldre C, Delfiore G, Peters JM, Sluse F, Crielaard JM. A positron emission tomography study of voluntarily and electrically contracted human quadriceps. Muscle Nerve 1997; 20(4): 505-507.
Vanderthommen M, Depresseux JC, Dauchat L, Degueldre C, Croisier JL, Crielaard JM. Spatial distribution of blood flow in electrically stimulated human muscle: a positron emission tomography study. Muscle Nerve 2000; 23(4): 482-489.
Vanderthommen M, Depresseux JC, Dauchat L, Degueldre C, Croisier JL, Crielaard JM. Blood flow variation in human muscle during electrically stimulated exercise bouts. Arch Phys Med Rehabil. 2002; 83(7): 936-941. doi: 10.1053/apmr.2002.33226.
Vicini P, Bonadonna RC, Utriainen T, Nuutila P, Raitakari M, Yki-Järvinen H, Cobelli C. Estimation of blood flow heterogeneity distribution in human skeletal muscle from positron emission tomography data. Ann Biomed Eng. 1997; 25(5): 906-910. doi: 10.1007/BF02684175.
Wagenmakers AJ, Strauss JA, Shepherd SO, Keske MA, Cocks M. Increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: effect of obesity and ageing. J Physiol. 2016; 594(8): 2207-2222. doi: 10.1113/jphysiol.2014.284513.
Wolfram RM, Budinsky AC, Sinzinger H. Assessment of peripheral arterial vascular disease with radionuclide techniques. Semin Nucl Med. 2001; 31(2): 129-142. doi: 10.1053/snuc.2001.21267.
Zacchigna S, Tasciotti E, Kusmic C, Arsic N, Sorace O, Marini C, Marzullo P, Pardini S, Petroni D, Pattarini L, Moimas S, Giacca M, Sambuceti G. In vivo imaging shows abnormal function of vascular endothelial growth factor-induced vasculature. Hum Gene Ther. 2007; 18(6): 515-524. doi: 10.1089/hum.2006.162.
Tags: Perfusion, Radiowater, Skeletal muscle
Updated at: 2019-02-19
Created at: 2016-11-10
Written by: Vesa Oikonen