Quantification of [68Ga]DOTATOC PET
[68Ga]DOTATOC ([68Ga]DOTA-(Tyr3)-octreotide) is a somatostatin analog which binds to subtypes 2 and 5 of somatostatin receptors (SSTR2 and SSTR5, respectively). Somatostatin receptors can be targeted in neuroendocrine tumour (NET) imaging. [90Y]DOTATOC has been proposed for targeted radionuclide therapy of human gliomas. Uptake of [68Ga]DOTATOC is high also in certain normal tissues, such as spleen (Kulkarni et al., 2013). Macrophages express SSTR2, and [68Ga]DOTATOC may therefore be useful in inflammation imaging. The tracer has specific uptake in atherosclerotic plaques (Li et al., 2013), and uptake in thoracic aorta correlates with cardiovascular risk factors (Lee et al., 2018). In atherosclerosis patients, [68Ga]DOTATATE discriminates high-risk versus low-risk coronary lesions better than [18F]FDG, and offers good image quality (Tarkin et al., 2017).
SSTR tumour imaging with [68Ga]DOTATOC is improved with pre-dose of unlabelled octreotide, suggesting that discontinuation of somatostatin analog therapy before PET/CT is not needed (Lodge et al., 2021).
Arterial plasma
Retrieving input function from [68Ga]DOTATOC studies is relatively easy, because correction for radioactive metabolites is not necessary, not even in rat studies. If arterial blood curve is measured from dynamic PET image IDIF), it needs to be converted to plasma (Menda et al., 2013), which can be done using haematocrit because [68Ga]DOTATOC does not pass red blood cell membranes. Alternatively, arterial blood curve has been directly used as model input (Koukouraki et al., 2006a and 2006b), in which case the results are biased by the blood-plasma conversion factor. This must be taken into account when comparing numerical results from different sites.
Receptor binding
Receptor binding can be quantitated using 2-tissue compartmental model (Henze et al., 2005; Koukoraki et al., 2006a; Menda et al., 2013), where the first tissue compartment represents specific (and possibly nonspecific) binding to receptors and the second tissue compartment represents internalized tracer-receptor complex (Heidari et al., 2013). Parameter ratios K1/k2 and k3/k4 are of special interest.
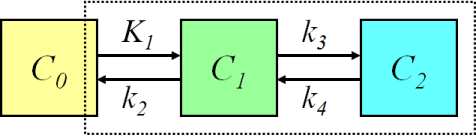
Because the bound radioligand is internalized into the cells, k2 and k4 may be very low with this radioligand (Henze et al., 2005). This may lead to difficulties in the fitting of the 2-tissue compartmental model, and therefore Logan plot could be a practical alternative for the analysis, if uptake actually is reversible. Heidari et al (2013) used Patlak plot successfully to analyse [68Ga]DOTATOC PET mice data, and Velikyan et al (2014) proposed its use in human studies. Therefore, it may be that uptake is in fact irreversible, and that the apparent k4>0 is only an artefact, which could be caused by e.g. tissue heterogeneity.
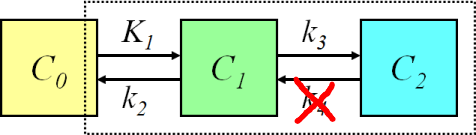
Semiquantitative methods (SUV and tumour-to-background ratios) are also widely used. It should be noted that since the uptake of [68Ga]DOTATOC in the normal spleen is very high, the SUV may not be comparable in cases of abnormalities of the spleen, and especially if spleen has been removed (Kratochwil et al., 2015). Tissue-to-blood ratio can be calculated easily when the heart or thoracic aorta is in the image.
See also:
Literature
Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, Herth F, Koukouraki S, Mäcke HR, Haberkorn U, Strauss LG. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using 68Ga-DOTATOC PET and comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2006; 33(7): 823-830. doi: 10.1007/s00259-005-0063-5.
Heidari P, Wehrenberg-Klee E, Habibollahi P, Yokell D, Kulke M, Mahmood U. Free somatostatin receptor fraction predicts the antiproliferative effect of octreotide in a neuroendocrine tumor model: implications for dose optimization. Cancer Res. 2013; 73(23): 6865-6873. doi: 10.1158/0008-5472.CAN-13-1199.
Koukouraki S, Strauss LG, Georgoulias V, Schuhmacher J, Haberkorn U, Karkavitsas N, Dimitrakopoulou-Strauss A. Evaluation of the pharmacokinetics of 68Ga-DOTATOC in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging 2006; 33(4): 460-466. doi: 10.1007/s00259-005-0006-1.
Menda Y, Ponto LL, Schultz MK, Zamba GK, Watkins GL, Bushnell DL, Madsen MT, Sunderland JJ, Graham MM, O'Dorisio TM, O'Dorisio MS. Repeatability of gallium-68 DOTATOC positron emission tomographic imaging in neuroendocrine tumors. Pancreas 2013; 42(6): 937-943. doi: 10.1097/mpa.0b013e318287ce21.
Velikyan I, Sundin A, Sörensen J, Lubberink M, Sandström M, Garske-Román U, Lundqvist H, Granberg D, Eriksson B. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014; 55(2): 204-210. doi: 10.2967/jnumed.113.126177.Zhang H, Moroz MA, Serganova I, Ku T, Huang R, Vider J, Maecke HR, Larson SM, Blasberg R, Smith-Jones PM. Imaging expression of the human somatostatin receptor subtype-2 reporter gene with 68Ga-DOTATOC. J Nucl Med. 2011; 52: 123-131. doi: 10.2967/jnumed.110.079004.
Tags: Somatostatin, SSTR, NET
Updated at: 2022-12-07
Created at: 2014-01-19
Written by: Vesa Oikonen